- How to choose an ozone generator
- About ozone
- coronavirus
- Water ozonation
- Ozonation of fruit and vegetables
- Contact
- Vehicle ozonation
- Ozone concentration
- Ways of ozonation
- The use of ozone in industry
- Air Ozonizers
- Ozonation of cereals
- Ozone treatment in the meat industry
- Ozonation in cold rooms
- Fire - ozonation
- Ozone breakdown in water
- Allergy
- Ozone compatible materials
- Decontamination of air conditioning with ozone
- Ozone water treatment
- Home ozone generator
- Mushrooms
- Sewage ozonation
- Ozonation of hotel rooms
About ozone and ozonation
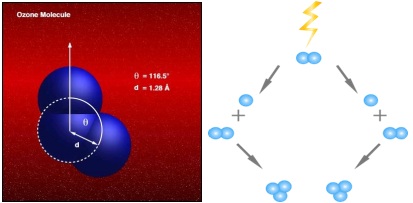
Ozone is a molecule consisting of three oxygen atoms (O3), with a delta negative and a delta positive electric charge. The ozone molecule is very unstable and has a short half-life. Therefore, it transforms relatively quickly into its original form, i.e. oxygen. In fact, ozone is nothing more than oxygen (O2) with an additional oxygen atom. An additional oxygen atom is created under the influence of an electric discharge as a result of the breakdown of an oxygen molecule. In nature, ozone is formed as a result of some chemical reactions. The most famous example is of course the so-called ozone layer, where ozone is formed under the influence of UV sun rays. Ozone is also formed during storms and near waterfalls. The characteristic "fresh, clean, spring" aroma of rain is the result of naturally formed ozone during a storm. Ozone comes from the Greek word ozein which means 'to smell'.
The image below shows how ozone destroys odors by oxidation.
Ozone is formed only in extreme conditions. They can be artificially created in so-called ozone generators also called ozonators. Ozone generators generate ozone as a result of electrical discharges or UV action.
Ozone works on the principle of oxidation. When a statically charged ozone molecule (O3) comes into contact with an oxidizable element, the ozone molecule immediately turns into oxygen. This is due to the fact that ozone is very unstable and quickly returns to its original form, i.e. oxygen (O2). Ozone can oxidize all types of materials, but also unpleasant odors and microorganisms such as viruses, molds and bacteria. During the operation of ozone, an additional oxygen atom is released from the molecule and it binds to the material that the ozone acts on. In the end, only a clean and stable oxygen molecule is formed.
Ozone is one of the strongest oxidants available to oxidize solutes. An additional atom in the ozone molecule binds (oxidizes) in a split second to each component that comes into contact with it.
Ozone production
Ozone can be artificially generated according to the principle on which it is formed in nature, i.e. using UV light (ozone layer) or by corona (high voltage, storms). In both methods, the bond between oxygen molecules is broken, where, as a consequence, oxygen radicals are formed, which connect to the oxygen molecule to give O3 (ozone). In ozone production, corona discharge is more often used due to the greater number of advantages of this method. These advantages include lower ozone production costs (more economically efficient) and longer system durability. Atmospheric air or pure oxygen may be used as the source gas. Oxygen concentrators (separators) to concentrate oxygen from air can be used to produce pure oxygen. Higher ozone concentration can be achieved if pure oxygen is used as the source gas, e.g. from oxygen cylinders.
The use of ozone
Ozone can be used in a wide range of cleaning and disinfection. It is used to the greatest extent for urban waste water treatment and drinking water production (disinfection). However, it is increasingly used in various industries. In the food industry, for example, ozone is used for disinfection, and in the paper and textile industry it is used for the oxidation of wastewater. The main advantage of ozone is its "clean" nature, because it oxidizes the material with virtually no by-products. Due to the fact that it has a strong recognizable odor, very low concentrations are immediately felt. This makes working with ozone safe.
In the purification of water and air, there is a need to produce ozone in the field. Due to its short half-life (half-life), ozone will decay practically as soon as it is produced. The half life in water is about 30 minutes, which means that every half hour the ozone concentration is reduced by half compared to the initial (previous) concentration. For example, when we have 8 mg / l, the concentration is reduced every half hour as follows: 8; 4; 2; 1; etc. In fact, the half life is even shorter because it can be affected by various factors. These factors are temperature, pH, ozone concentration and the concentration and type of other solutes. Due to the fact that ozone reacts with many other components, the ozone concentration drops rapidly. When most of the compounds are oxidized, an ozone residue is formed, the concentration of which does not drop so quickly.

In the case of a hotel room , which must be prepared for a new guest in a short time thanks to the use of an ozone generator, you can get rid of stale and unpleasant smell. Polluted air can be changed into full freshness smelling in less than 45 minutes, only by using the appropriate device. The use of an ozone generator is also able to disinfect the air in the room and its equipment, reducing the risk of bacterial or viral infection. Ozonation in addition to sterilizing the room, it also completely removes mold and fungi, as well as 90% removes all rodents, insects and pests.
Ozone is also most applicable in the production rooms of meat processing plants, where thanks to the sterilization of the atmosphere in the room, it greatly improves sanitary conditions. The raw material for production, as well as the finished product that is in the atmosphere with ozone content is resistant to bacterial or viral infections. Cars used for transporting meat and meat products are subjected to ozonation in order to eliminate the cell that allows contamination of goods. Ozonation of rooms where meat is cut, refrigerated counters, sales rooms in butcher shops, extends the freshness and durability of meat as well as its products as much as possible. In recent times, it is not without significance to notice in the meat stores a fresh smell devoid of the typical odor of perishable meat.
In fish processing, ozonation of rooms where fish are found completely eliminates odor and prolongs fish freshness.
In the case of animal husbandry, ozonation prevents infectious diseases as well as removes unpleasant odors from breeding.
Ozone is also used in swimming pools , so water ozonization sterilizes, removes algae, purifies water, and above all leads to full transparency of water in the tank.
Ozone is also used in hospitals, outpatient clinics, operating rooms, because thanks to it you can avoid infections and infections.
In the case of animal husbandry, ozonation prevents infectious diseases as well as removes unpleasant odors from breeding.
Ozone is also used in hospitals , outpatient clinics, operating rooms, because thanks to it you can avoid infections and infections.
Harmfulness of ozone
Ozone is harmful to human health if it is inhaled for a long time or in high concentrations. More than a dozen organizations, such as The Occupational Safety and Health Agency (OSHA) has proposed the maximum allowable (acceptable) concentration (MAC) for ozone. This value means the maximum concentration to which a person can be exposed at a given time and for a given relationship. For ozone, the MAC value is 0.06 PPM for 8 hours a day, 5 days a week (PPM = Pars Per Million). A maximum MAC value of 0.3 PPM is used for a maximum of 15 minutes. The aforementioned concentrations are much higher than the threshold value at which ozone may be perceptible in the air and the presence of ozone is perceptible in the air at low concentrations. When people are exposed to high levels of ozone, the symptoms can range from dry mouth and throat, coughing, to headache and chest pain. The closer the concentration is to the lethal dose, the more severe the symptoms appear.
According to the ordinance of the MINISTER OF FAMILY, WORK AND SOCIAL POLICY of June 12, 2018, the highest permissible concentration of ozone in the workplace according to PN – Z – 04007–2: 1994 is 0.15 mg / m3 during a work shift, i.e. for 8 hours of work.
Ozone measurement
There are many devices for measuring ozone concentrations in water and air. The operation of these devices is based on different principles and they can measure concentration from PPM (PPM = Parts Per Million) to PPB (= Parts Per Billion). These devices can be used to monitor and control ozone generators

Ozone concentration table.
DESCRIPTION | EXPOSURE TIME | PPM SIZE | FORMAT MG / M 3 |
Permissible ozone concentration at the workplace | 8 h | 0.05 - 0.1 ppm | 0.107 - 0.2 mg / m 3 |
Odor detection - medium |
| 0.02 ppm | 0.04 mg / m 3 |
Smell detection - the range depends on the body's properties |
| 0.01 - 0.04 ppm | 0.02 - 0.086 mg / m 3 |
Minimal concentration causing eye, nose, throat irritation, headache, shortness of breath |
| from 0.1 ppm | from 0.2 mg / m 3 |
Breathing problems, reduced oxygen uptake, breathing problems, general fatigue and chest pain, dry cough |
| 0.5 - 1.00 ppm | 1.07 - 2.14 mg / m 3 |
Headache, breathing disorders, drowsiness, severe pneumonia with longer exposure |
| 1 - 10 ppm | 2.14 - 21.4 mg / m 3 |
Danger to life and health |
| 10 ppm | 21.4 mg / m |
Lethal concentration for small animals within 2 hours |
| 15-20 ppm | 32.1 - 42.8 mg / m 3 |
Deadly concentration in a few minutes |
| above 1700 ppm | above 3 638 mg / m |
Advanced ozonation technology ensuring full product safety and simplicity of use, arouses more and more interest among a large number of companies considering purchasing these systems, whose technology has already gained wide recognition and trust of customers around the world.

